13. Fundamentals of Electrochemistry
Electrochemical Cells
13. Fundamentals of Electrochemistry
Electrochemical Cells
Practice this topic
- Open Question
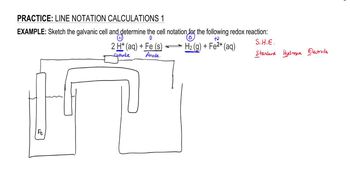
Sketch the galvanic cell and determine the line notation for the following redox reaction:
Ni2+ (aq) + Mg (s) ⇌ Ni (s) + Mg 2+ (aq)
114views - Multiple ChoiceCalculate the standard cell potential (E°cell) for the galvanic cell based on the following reaction: Zn(s) + Cu^2+(aq) → Zn^2+(aq) + Cu(s). Given: E°(Zn^2+/Zn) = -0.76 V, E°(Cu^2+/Cu) = +0.34 V.8views